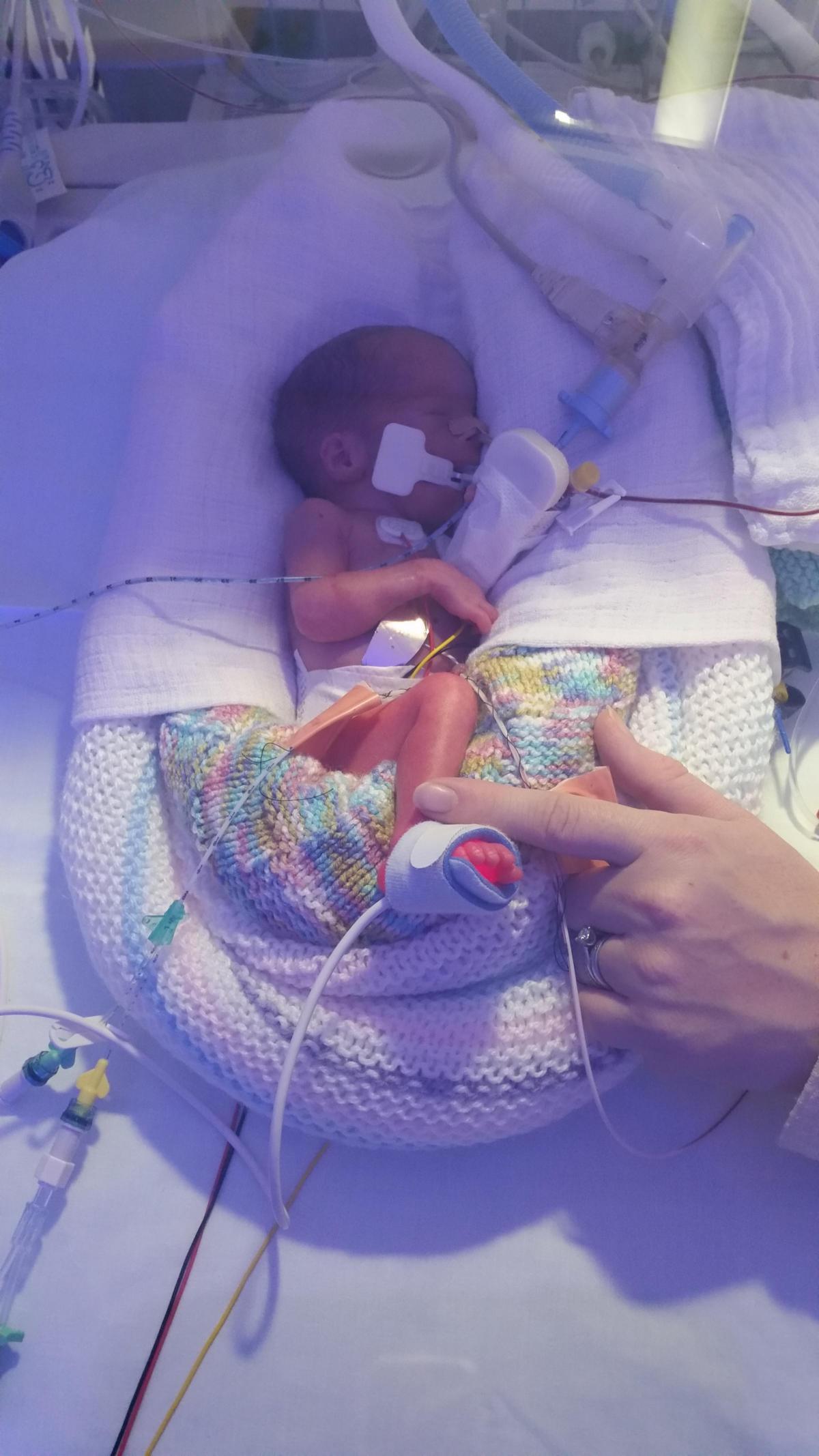
A BREAKTHROUGH new drug treatment which saved the life of a tiny twin born 12 weeks prematurely could soon be widely available.
Emily Kearey, who lives in Colchester, was born three months early along with sister Sophie in November 2014.
The brave pair were delivered early be caesarean after they developed twin-to-twin transfusion syndrome – a severe disorder of blood vessels in the shared placenta.
While Sophie tipped the scales at 3lb 3oz, little Emily only weighed 1lb 13oz and quickly showed signs of respiratory distress syndrome which affects premature babies due to their immature lungs and the lack of surfactant – a substance which covers tiny lung sacs.
Youngsters are usually treated extra surfactant derived from the lungs of pigs, but at the time the Neonatal Intensive Care Unit at Norfolk and Norwich University Hospital where the girls were born were part of an international clinical trial investigating synthetic surfactant CH5633.
The study was the first time it had been tested on humans and Emily was one of just 40 babies in Europe to take part.
Thankfully, she responded to the treatment and after two weeks on the neonatal unit, was allowed to go back to Colchester General Hospital and then back home.
Now more than two years later, lively toddler Emily has hit all her milestones and the findings on the trial have been published in the Archives of Disease in Childhood.
Dad Roger, who is an out of hours GP, said: “Working in healthcare we knew a little bit about clinical research and were happy for Emily to go into the study, but it was still a daunting decision to make.
“We had complete trust in the doctors and were confident that we had been provided with all the information necessary to make our decision.
“It was important for us to take part in this study, for Emily first and foremost, but also because we knew that this could help premature babies with similar difficulties in the future.
“We received brilliant care from the team at NNUH and are grateful for their expertise and support.”
The trial was led by Dr David Sweet, Chief Investigator for the study and consultant neonatologist at the Royal Maternity Hospital in Belfast.
Dr Sweet said: “This important study has shown the new synthetic surfactant CH5633 appears safe for use in premature babies and looks to be a promising new treatment when they have breathing difficulties.
“Our study paved the way for a much larger study now underway which is continuing to evaluate the effectiveness of CH5633.
“If this larger study confirms it to be an effective treatment, we hope it will eventually be made widely available to treat future premature babies born with breathing difficulties.”
Dr Paul Clarke, consultant neonatologist and principal investigator for the study at the hospital said the trial helped to confirm the hospital’s reputation as innovators.
He said: “We are most grateful to Emily and her parents for allowing her to participate.”




Comments: Our rules
We want our comments to be a lively and valuable part of our community - a place where readers can debate and engage with the most important local issues. The ability to comment on our stories is a privilege, not a right, however, and that privilege may be withdrawn if it is abused or misused.
Please report any comments that break our rules.
Read the rules here